Abstract
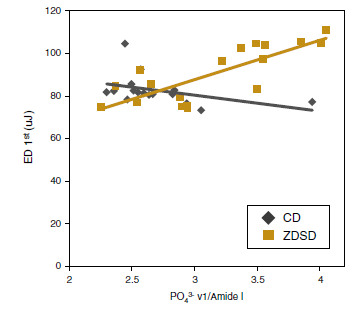
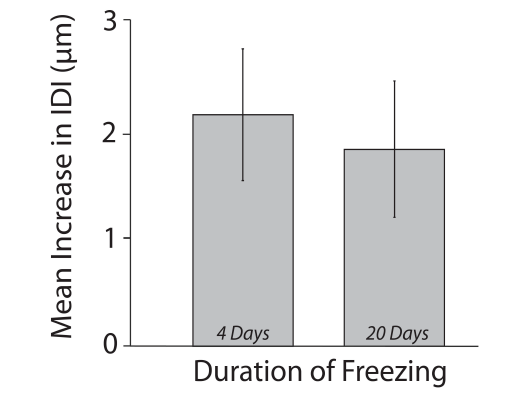
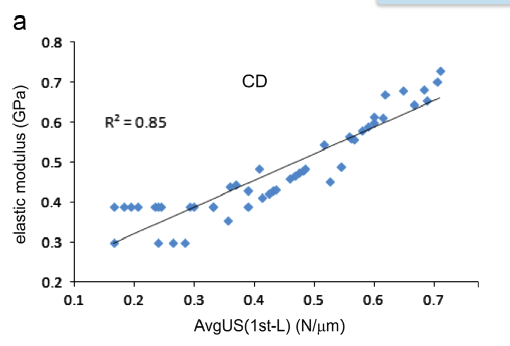
Paget’s disease of bone (PDB) is the second most common bone disease mostly developing after 50 years of age at one or more localized skeletal sites; it is associated with severely high bone turnover, bone enlargement, bowing/deformity, cracking, and pain. Here, to specifically address the origins of the deteriorated mechanical integrity, we use a cohort of control and PDB human biopsies to investigate multiscale architectural and compositional modifications to the bone structure (ie, bone quality) and relate these changes to mechanical property measurements to provide further insight into the clinical manifestations (ie, deformities and bowing) and fracture risk caused by PDB. Here, at the level of the collagen and mineral (ie, nanometer-length scale), we find a 19% lower mineral content and lower carbonate-to-phosphate ratio in PDB, which accounts for the 14% lower stiffness and 19% lower hardness promoting plastic deformation in pathological bone. At the microstructural scale, trabecular regions are known to become densified, whereas cortical bone loses its characteristic parallel-aligned osteonal pattern, which is replaced with a mosaic of lamellar and woven bone. Although we find this loss of anisotropic alignment produces a straighter crack path in mechanically-loaded PDB cases, cortical fracture toughness appears to be maintained due to increased plastic deformation. Clearly, the altered quality of the bone structure in PDB affects the mechanical integrity leading to complications such as bowing, deformities, and stable cracks called fissure fractures associated with this disease. Although the lower mineralization and loss of aligned Haversian structures do produce a lower modulus tissue, which is susceptible to deformities, our results indicate that the higher levels of plasticity may compensate for the lost microstructural features and maintain the resistance to crack growth.
https://www.ncbi.nlm.nih.gov/pubmed/25112610
J Bone Miner Res. 2015 Feb;30(2):264-73. doi: 10.1002/jbmr.2340.